Glucose
Glucose bụ shuga nwere usoro . Glucose bụ n'ozuzu ya monosaccharide kachasị, [4] otu subcategory nke carbohydrates. Glucose na-abụkarị nke osisi na ọtụtụ algae n'eme n'oge photosynthesis site na mmiri na carbon dioxide, n'eji ike sitere na ìhè anyanwụ, ebe a n'eji ya eme cellulose na mgbidi mkpụrụ ndụ, carbohydrate kachasị n'ụwa.[5][6][7]
 Usoro d="13" href="./Skeletal_formula" rel="mw:WikiLink" title="Skeletal formula">Ọkpụkpụ nke d-glucose
| |
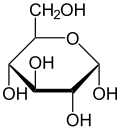 Nkọwa Haworth nke α-d-glucopyranose
| |
 Nkọwapụta nke d-glucose
| |
| Aha | |
|---|---|
| Nkwupụta | //<span style="border-bottom:1px dotted"><span title="/ˈ/: primary stress follows">'</span><span title="/ɡ/: 'g' in 'guy'">ɡọ</span><span title="'l' in 'lie'">l</span><span title="/uː/: 'oo' in 'goose'">uː</span><span title="'k' in 'kind'">k</span><span title="/oʊ/: 'o' in 'code'">Oʊ</span><span title="'z' in 'zoom'">z</span></span>//, //<span style="border-bottom:1px dotted"><span title="/ɡ/: 'g' in 'guy'">ɡọ</span><span title="'l' in 'lie'">l</span><span title="/uː/: 'oo' in 'goose'">uː</span><span title="'k' in 'kind'">k</span><span title="/oʊ/: 'o' in 'code'">Oʊ</span><span title="'s' in 'sigh'">s</span></span>// |
| Aha IUPAC Aha ndị a na-enye ohere: [1]
| |
| Aha IUPAC kachasị amasị A naghị amata PIN maka ngwaahịa okike. | |
Aha usoro IUPAC
| |
| Aha ndị ọzọ Ọbara shugaDextroseCorn shuga-GlucoseGrape shuga
| |
| Ihe ndị na-eme ka a mata ya | |
Nọmba CAS
|
Page Templeeti:Plainlist/styles.css has no content.
|
Ihe nlereanya 3D (JSmol)
|
Page Templeeti:Plainlist/styles.css has no content. |
| 3DMet | Page Templeeti:Plainlist/styles.css has no content. |
| Nchịkọta | Glc |
Nkwupụta Beilstein
|
1281604 |
| ChEBI | Page Templeeti:Plainlist/styles.css has no content. |
| ChEMBL | Page Templeeti:Plainlist/styles.css has no content. |
| ChemSpider | Page Templeeti:Plainlist/styles.css has no content. |
| <span title="European Community number (chemical identifier)">Nọmba EC</span> | Page Templeeti:Plainlist/styles.css has no content.
|
Nkwupụta Gmelin
|
83256 |
IUPHAR/BPS
|
Page Templeeti:Plainlist/styles.css has no content. |
| KEGG | Page Templeeti:Plainlist/styles.css has no content. |
| MeSH | Glucose |
PubChem <abbr title="<nowiki>Compound ID</nowiki>">CID
|
Page Templeeti:Plainlist/styles.css has no content. |
| Nọmba RTECS | Page Templeeti:Plainlist/styles.css has no content.
|
| UNII | Page Templeeti:Plainlist/styles.css has no content.
|
InChI
| |
Udo
| |
| Ihe onwunwe | |
Usoro kemịkalụ
|
C6H12O6 |
| Ọkpụkpụ nke ọkpụkpụ | 180.156 g/mol |
| Ọdịdị | Ọcha ọcha |
| Ọnụ ọgụgụ mmadụ | 1.54 g/cm3 |
| Ebe ọ na-agbaze | α-d-Glucose: 146 °C (295 °F; 419 K) β-d-Clucose: 150 °C (302 °F; 423 K) d |
Ọdịdị mmiri
|
909 g/L (25 °C (77 °F)) |
Mmetụta magnetik (χ)
|
-101.5×10−6 cm3/mol |
Oge Dipole
|
8.6827 |
| Okpomọkụ na kemịkalụ | |
Ikike okpomọkụ (C)
|
218.6 J/ (K·mol) [2] |
Std molarentropy<br> (Sjá 298) (Ọ dị n'afọ 298)
|
209.2 J/ (K·mol) [2] [2] |
Std enthalpy offormation (ΔfHZ298) (Dị ka nke a na-akpọ, 298)
|
-1271 kJ/mol[3] |
Okpomọkụ nke ọkụ, uru dị elu (HHV)
|
2,805 kJ/mol (670 kcal/mol) |
| Ọgwụ na-ahụ maka ọgwụ | |
Koodu ATC
|
B05CX01 (WHO) V04CA02 (WHO), V06DC01 (WHO). |
| Ihe ize ndụ | |
| <b>NFPA 704</b> (diamond ọkụ) | Page Templeeti:NFPA 704 diamond/styles.css has no content. <span title="Health 0: Exposure under fire conditions would offer no hazard beyond that of ordinary combustible material. E.g. sodium chloride">0</span> <span title="Flammability 1: Must be pre-heated before ignition can occur. Flash point over 93 °C (200 °F). E.g. canola oil">1</span> <span title="Instability 0: Normally stable, even under fire exposure conditions, and is not reactive with water. E.g. liquid nitrogen">0</span> |
| Akwụkwọ data nchekwa (SDS) | ICSC 08655 |
Ewezuga ebe a kpọtụrụ aha, a na-enye data maka ihe dị na ọnọdụ ha (na 25 °C [77 °F], 100 kPa). Ihe odide Infobox
| |
Na metabolism ike, glucose bụ isi iyi kachasị mkpa nke ike na ihe niile dị ndụ. A n'echekwa glucose maka metabolism dị ka polymer, na osisi dịka starch na amylopectin, na anụmanụ dị ka glycogen. Glucose naagagharị n'ọbara ụmụ anụmanụ dị ka shuga dị n'ọgbụgba.[5][7] Ndammana glucose n'apụta n'okike bụ D-glucose, ebe a n'emepụta stereoisomer ya -glucose n'ụzọ aka na obere ma ọ naghị arụ ọrụ nke ọma.[2] Glucose bụ monosaccharide nwere atọm carbon isii na otu aldehyde, ya mere ọ bụ aldohexose. Mkpụrụ ndụ glucose nwere ike ịdị n'ụdị oghere (acyclic) yana ụdị mgbanaka (cyclic). Glucose n'eme n'ụzọ okike ma n'ahụ ya n'ọnọdụ ya n'efu na mkpụrụ osisi na akụkụ ndị ọzọ nke osisi. N'ime ụmụ anụmanụ, a n'ewepụta glucose site na mmebi nke glycogen na usoro a maara dị ka glycogenolysis.
Glucose, dị ka ihe ngwọta shuga intravenous, dị na Ndepụta Ọgwụ Ndị Dị Mkpa nke Òtù Ahụ Ike Ụwa.[8] Ọ dịkwa na ndepụta ahụ na njikọta ya na sodium chloride (mmanụ nnu). [1][8]
Aha glucose sitere na Grik oge ochie γλεῦκος (gleûkos, "vine, must"), site na γλυκύς (glykýs, "sweet").[9] Ntinye "-ose" bụ onye na-ekewa kemịkal na-egosi shuga.
Mmetụta glucose dị n'ahụ mmadụ
Glucose bụ isi iyi ike dị mkpa maka ahụike mmadụ. N'ime ahụ na-arụ ọrụ, sel na-amịkọrọ glucose site na nri iji mepụta ume. Usoro a na-emepụta molekul ume a na-akpọ ATP. ATP bụ isi iyi ike dị mkpa maka arụ ọrụ nkịtị nke akwara, ụbụrụ na akụkụ ahụ ndị ọzọ. Na mgbakwunye, glucose na-akwalite nchekwa ike na ọrụ mkpụrụ ndụ ọbara uhie site na homonụ na-achịkwa ọrụ ụbụrụ na ọkwa shuga ọbara. Isi iyi a bụ otu n'ime ụmụ irighiri ihe dị mkpa maka ndụ ma dị mkpa maka ahụike mmadụ.
Ọnụego glucose ọbara a na-ahazi maka mmadụ dị n'etiti 70 na 100 mg / dl.
Ekwesịrị ịlele ọkwa glucose n'ụtụtụ mgbe emepere emepe. Ndị mmadụ karịrị afọ 40 ka a na-atụ aro ka ha nyochaa glucose kwa afọ 3.
Nkwado glucose
Nkwado glucose, nke bụ ụzọ na usoro dị iche iche iji mee ka oriri glucose na-eri nri ma ọ bụ ịnọgide na-enwe ọkwa shuga dị n'ọbara. Ụfọdụ ụzọ a na-ahụkarị gụnyere ndị a (ịkwesịrị ka gị na dọkịta gị na-ekwurịta nkwado glucose mgbe niile):
- Nri: Ị nwere ike imetụta ọ̀tụ̀tụ̀ shuga dị n'ọbara gị site n'iri ụdị nri ụfọdụ.
- Carbohydrates. Carbohydrates dị mgbagwoju anya na nri nwere ike inye glucose na-ahapụ ngwa ngwa ma nyere aka ịnọgide na-enwe ọkwa shuga dị n'ọbara. Ndị a na-agụnye nri ndị nwere carbohydrates dị mgbagwoju anya, dị ka ngwaahịa ọka wit na mkpụrụ osisi na akwụkwọ nri na-esighị ike.
- Ihe mgbakwunye: Dabere na ọtụtụ ahụmịhe ụlọ ọgwụ, emepụtara ọtụtụ ihe inyeaka mgbakwunye. Ha nwere ike ịnwe ihe ndị sitere n'okike na-emetụta ọkwa shuga dị n'ọbara. Ihe mgbakwunye dị otú ahụ abụghị ọgwụ, n'ihi ya, ha anaghị agwọ ọrịa. Ị kwesịrị ịkpọtụrụ dọkịta gị mgbe niile tupu ị na-eji ha.
- Nnyocha ahụike na ọgwụgwọ. A na-enweta ọgwụgwọ na nyocha ahụike kwesịrị ekwesị iji jikwaa ma kwado ọkwa glucose. Ndị a gụnyere usoro ọgwụgwọ ọgbara ọhụrụ na usoro iji chịkwaa ọkwa shuga dị n'ọbara ma mee ka ndị nwere ọrịa shuga dị mma.
- Insulin na ọgwụ antidiabetic. A na-enye ndị nwere ọrịa shuga insulin ma ọ bụ ọgwụ antidiabetic iji mezie ọkwa shuga dị n'ọbara ha ka ọ dị mkpa. A na-eji ọgwụ ndị a emezigharị n'otu n'otu iji kwado na ịhazi ọkwa shuga dị n'ọbara.
Akụkọ ihe mere eme
dezieGlucose bụ nke mbụ onye Germany n'emepụta kemịkal Andreas Marggraf wepụtara site na Mkpụrụ vaịn n'afo 1747. [10] Glucose chọpụtara na mkpụrụ vaịn site n'aka onye Germany ọzọ na kemịkal Johann Tobias Lowitz - n'afo1792, ma mee ka ọ dị iche na shuga okpete (sucrose). Glucose bụ okwu nke Jean Baptiste Dumas chepụtara n'afo 1838, nke jupụtara na akwụkwọ kemịkal. Friedrich August Kekulé chepụtara okwu dextrose (site na Latịn dexter, nke pụtara "aka nri"), n'ihi na na mmiri nke glucose, a n'atụgharị ụgbọ elu nke ìhè polarized n'aka nri. N'ụzọ ="smallcaps" data-ve-ignore="true" id="mwbA">d iche, l-fructose (nke a na-akpọkarị d-fructosis) (a ketohexose) na l-glucose (l-glucose) na-agbanwe ìhè polarized n'ụzọ kwụ ọtọ n'aka ekpe. Nkọwa mbụ dị ka ntụgharị nke ụgbọ elu nke ìhè polarized (d na l-nomenclature) ka e mesịrị hapụ maka d- na l-notation, nke na-ezo aka na nhazi zuru oke nke etiti asymmetric kacha ="smallcaps" data-ve-ignore="true" id="mwdw">d anya site na otu carbonyl, yana n'ikwekọ na nhazi nke d- ma ọ bụ l-glyceraldehyde.[11]
Ebe ọ bụ na glucose bụ ihe dị mkpa nke ọtụtụ ihe ndị dị ndụ, nghọta ziri ezi banyere ihe ndị mejupụtara ya na ọdịdị ya nyere aka nke ukwuu na ọganihu n'ozuzu ya na kemịkalụ kemịkal. Nghọta a mere n'ụzọ dị ukwuu n'ihi nyocha nke Emil Fischer, onye Germany n'emepụta kemịkal nke natara onyinye Nobel nke afo 1902 na kemịkal maka nchọpụta ya. Njikọ nke glucose guzobere ọdịdị nke ihe ndị dị ndụ ma si otú a mepụta nkwenye mbụ nke Jacobus Henricus van 't Hoff's echiche nke kemịkalụ kinetics na nhazi njikọ kemịkalụ na ụmụ irighiri ihe n'ebu Kabon . N'etiti n'afo 1891 na 1894, Fischer guzobere nhazi stereochemical nke shuga niile a maara ma buru amụma nke ọma banyere isomers nwere ike, n'etinye echiche Van 't Hoff nke atọm Kabon asymmetrical. Aha ndị ahụ na mbụ n'ezo aka na ihe ndị sitere n'okike. E nyere enantiomers ha otu aha ahụ site na iwebata aha aha usoro, na-eburu n'uche stereochemistry zuru oke (="smallcaps" data-ve-ignore="true" id="mwig">d Fischer nomenclature, d / l nomenclatures).
Maka nchọpụta nke metabolism nke glucose Otto Meyerhof natara ihe nriya Nobel na nká mmụta Uche ma ọ bụ Ogwụ n'afo 1922. E nyere Hans von Euler-Chelpin ihe nrite Nobel na kemistri ya na Arthur Harden n'afo 1929 maka "nnyocha ha gbasara ịgba shuga na òkè ha na enzymes na usoro a". N'afọ 1947, Bernardo Houssay (maka nchọpụta ya banyere ọrụ nke pituitary gland na metabolism nke glucose na carbohydrates ndị sitere na ya) yana Carl na Gerty Cori (maka nchoputa ha banyere ntụgharị nke glycogen site na glucose) natara onyinye Nobel na nká mmụta uche ma ọ bụ Ogwụ. N'afọ 1970, e nyere Luis Leloir ihe nrite Nobel na Kemistri maka nchọpụta nke glucose eweputara na shugar nucleotides na biosynthesis nke carbohydrates.
Ihe onwunwe kemịkal na nke anụ ahụ
dezieGlucose na-emepụta ihe n'acha ọcha ma ọ bụ n'enweghị ụcha nke n'agbaze nke ukwuu na mmiri na acetic acid mana anaghị agbaze nke ọma na ethanol" id="mwqA" rel="mw:WikiLink" title="Methanol">methanol na ethanol. Ha n'agbaze na afo146 (α) na 150 "beta), n'agbaji malite na 188 na ntọhapụ nke ngwaahịa dị iche iche n'agbapụ agbapụ, n'ikpeazụ n'ahapụ ihe fọdụrụ na carbon. Glucose nwere uru pKa nke 12.16 na 25 °F) na mmiri.[12]
With six carbon atoms, it is classed as a hexose, a subcategory of the monosaccharides. Templeeti:Smd-Glucose is one of the sixteen aldohexose stereoisomers. The Templeeti:Sm-isomer, Templeeti:Sm-glucose, also known as dextrose, occurs widely in nature, but the Templeeti:Sm-isomer, <span data-cx="[{"adapted":false,"targetExists":false}]" data-mw="{"parts":[{"template":{"target":{"wt":"sm","href":"./Template:Sm"},"params":{"1":{"wt":"l"}},"i":0}}]}" data-ve-no-generated-contents="true" id="mwxw" typeof="mw:Transclusion"> </span>-glucose, does not. Glucose can be obtained by hydrolysis of carbohydrates such as milk sugar (lactose), cane sugar (sucrose), maltose, cellulose, glycogen, etc. Dextrose is commonly commercially manufactured from cornstarch in the US and Japan, from potato and wheat starch in Europe, and from tapioca starch in tropical areas. The manufacturing process uses hydrolysis via pressurized steaming at controlled pH in a jet followed by further enzymatic depolymerization. Unbonded glucose is one of the main ingredients of honey.[13][14][15][16][17]
- ↑ Nomenclature of Carbohydrates (Recommendations 1996) | 2-Carb-2 Archived 2023-08-27 at the Wayback Machine. iupac.qmul.ac.uk.
- ↑ 2.0 2.1 Boerio-Goates J (1991), "Heat-capacity measurements and thermodynamic functions of crystalline α-D-glucose at temperatures from 10K to 340K", J. Chem. Thermodyn., 23 (5): 403–09, doi:10.1016/S0021-9614(05)80128-4
- ↑ Ponomarev VV, Migarskaya LB (1960), "Heats of combustion of some amino-acids", Russ. J. Phys. Chem. (Engl. Transl.), 34: 1182–83
- ↑ Domb (1998-02-04). Handbook of Biodegradable Polymers. CRC Press. ISBN 978-1-4200-4936-7.
- ↑ 5.0 5.1 NCATS Inxight Drugs — DEXTROSE, UNSPECIFIED FORM. Archived from the original on 2023-12-11. Retrieved on 2024-03-18.
- ↑ Kamide (2005). Cellulose products and Cellulose Derivatives: Molecular Characterization and its Applications, 1st, Amsterdam: Elsevier. ISBN 978-0-08-045444-3. Retrieved on 13 May 2021.
- ↑ 7.0 7.1 L-glucose (en-US). Biology Articles, Tutorials & Dictionary Online (2019-10-07). Archived from the original on 2022-05-25. Retrieved on 2022-05-06.
- ↑ 8.0 8.1 (2019) World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ Online Etymology Dictionary. Etymonline.com. Archived from the original on 2016-11-26. Retrieved on 2016-11-25.
- ↑ (2015) Encyclopedia of Food and Health (in en). Academic Press. ISBN 978-0-12-384953-3.
- ↑ Rosanoff (1906). "On Fischer's Classification of Stereo-Isomers.1". Journal of the American Chemical Society 28: 114–121. DOI:10.1021/ja01967a014. Retrieved on 2019-07-01.
- ↑ Bosch (2004). "Binary and ternary phenylboronic acid complexes with saccharides and Lewis bases". Tetrahedron 60 (49): 11175–11190. DOI:10.1016/j.tet.2004.08.046. ISSN 0040-4020.
- ↑ (2023) "Comprehensive review on functional and nutraceutical properties of honey". Efood 4 (2). DOI:10.1002/efd2.71.
- ↑ (2018) "Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes". Oxidative Medicine and Cellular Longevity 2018: 1–12. DOI:10.1155/2018/4757893. PMID 29507651.
- ↑ (2020) "Scope of Honey in Diabetes and Metabolic Disorders", Therapeutic Applications of Honey and its Phytochemicals. Springer, 195–217. DOI:10.1007/978-981-15-7305-7_9. ISBN 978-981-15-7304-0. Retrieved on 2024-03-18.
- ↑ (2010) "Contribution of honey in nutrition and human health: A review". Mediterranean Journal of Nutrition and Metabolism 3: 15–23. DOI:10.1007/s12349-009-0051-6. Retrieved on 2024-03-18.
- ↑ (2006) "US Honeys Varying in Glucose and Fructose Content Elicit Similar Glycemic Indexes - Journal of the American Dietetic Association". Journal of the American Dietetic Association 106 (8): 1260–1262. DOI:10.1016/j.jada.2006.05.003. PMID 16863724.